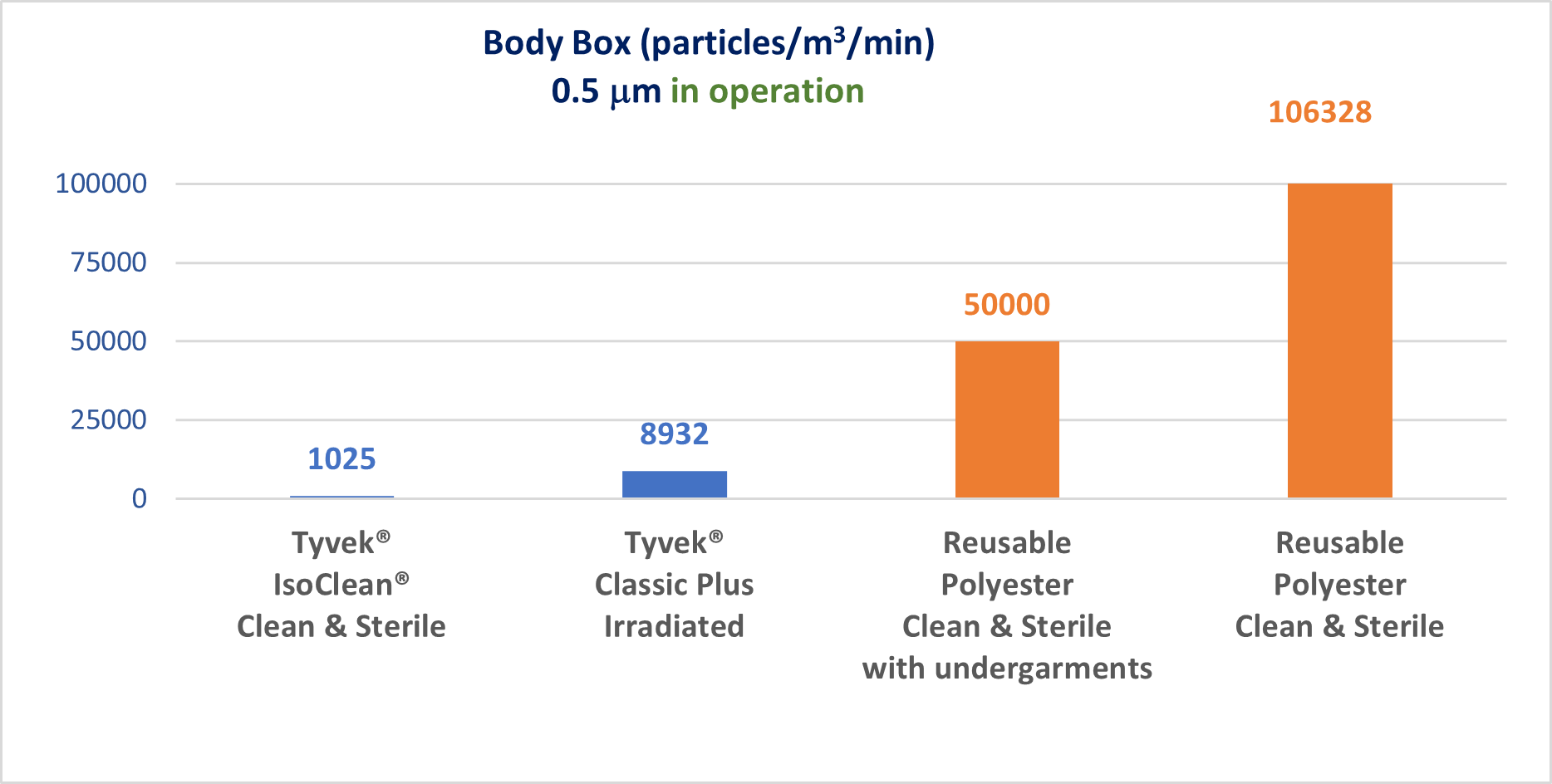
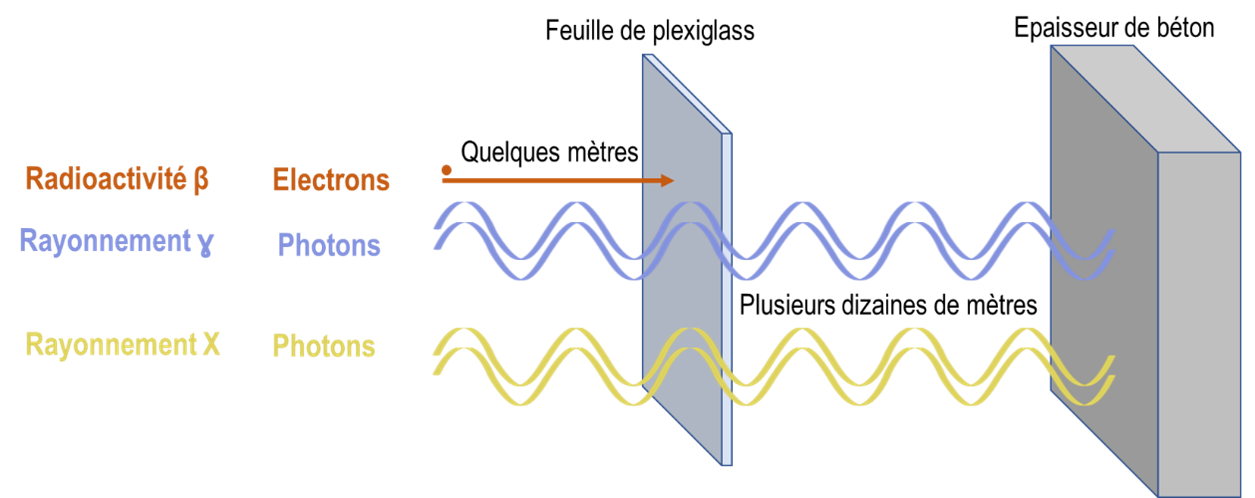
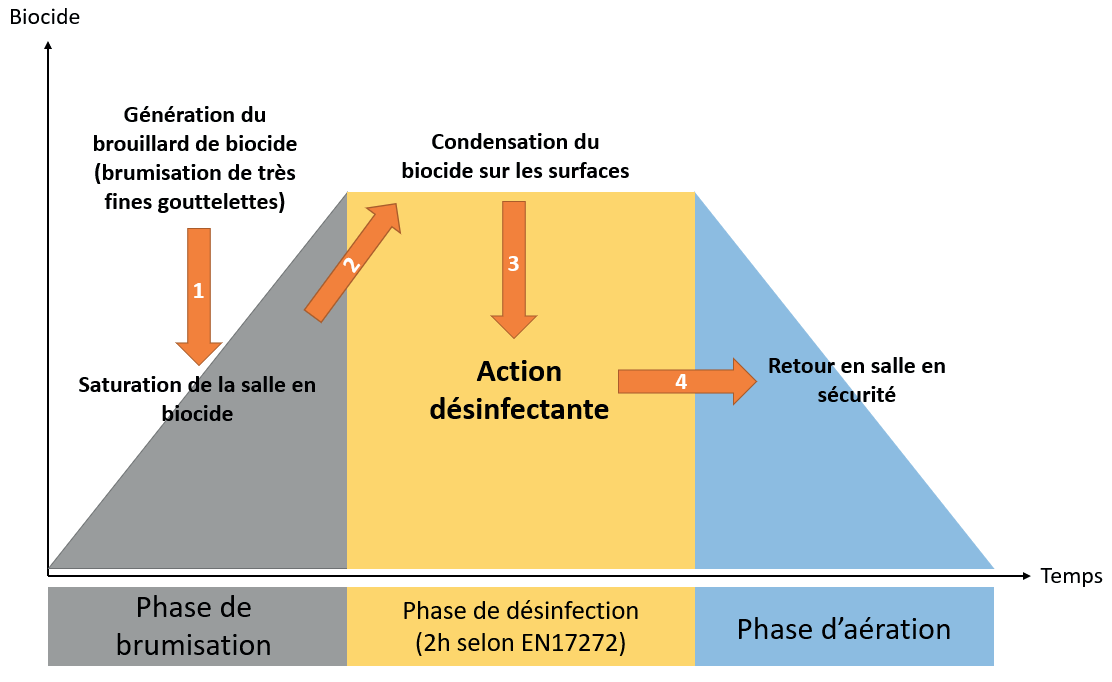
By the summer of 2023, all manufacturers of sterile healthcare products (active substances, excipients, primary packaging materials and finished galenic forms) will have to comply with the new recommendations in Annex 1 of the GMP (Good Manufacturing Practices).
This revision of Annex 1 makes Quality Risk Management the pivotal element in the control of contamination, whether microbiological, particulate or chemical.